Hey! Who’s a fan of organic chemistry? *crickets* OK, you may not have liked studying it, but without those chains of carbon-based molecules life would be rough. So here at Protolabs we’re all about paying our respects to those special groups of bonded atoms called polymers. This is especially true because the plastics we use in manufacturing are all based on chains of carbon molecules and their chemical structure affects their strength, the way that they interact with the environment, their appearance, and more. But this isn’t an org chem course. Nope. It’s a blog post to help you understand how different chains of organic molecules affect the material you choose to make your parts. So, gimme a C (for Carbon)!
Monomers: The Building Blocks of Plastics
Monomers are molecules that want to bond together with other molecules, when they do, they form chains called polymers, kind of like string cheese. Not surprisingly, that process is called polymerization. For the sake of simplicity, it’s enough to know that homopolymers are chains containing long strands of a single type of monomer. Copolymers are made up of two or more different types of bonded monomers.
Without monomers, there would be no plastics. Some common ones are ethylene, propylene, styrene, formaldehyde, phenol, acetonitrile, and ethylene glycol.
Chemical Comparison of Homopolymer vs. Copolymer
|
Characteristic |
Homopolymer |
Copolymer |
|
Chemical makeup |
Single repeating monomer |
Two or more monomers |
|
Structure |
Simple chain |
Complex chain |
|
Examples |
PVC, polyethylene, polypropylene, polystyrene |
Poly (vinyl acetate), poly (ethylene oxide) |
For example, PVC, the white piping you probably see all around your basement, is made up of monomers of just one type—vinyl chloride—that have chained together to become polyvinyl chloride. The “poly” part means “many.” The copolymer nylon 6/6 is a combination of two monomers: adipic acid and hexamethylenediamine.
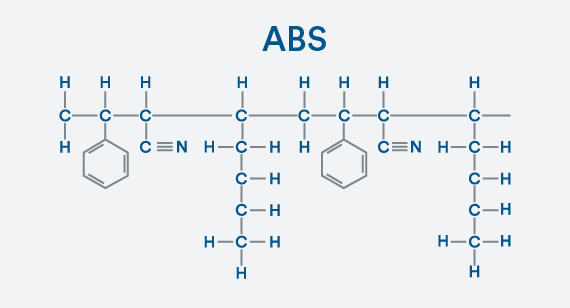
Building a Copolymer
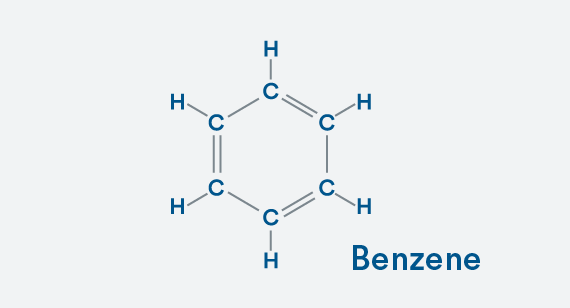
Now, here’s the info that will dazzle companions on first dates. It’s a basic example of how you move from a simple molecule to a much more complex copolymer (see illustration). Start with a basic hydrocarbon—a benzene ring. Each carbon atom needs four bonds to achieve stability, and you can see that benzene has that as each carbon atom is bonded once to one carbon atom and twice to its other carbon neighbor. To complete the balanced molecule, we add a hydrogen atom to each carbon.
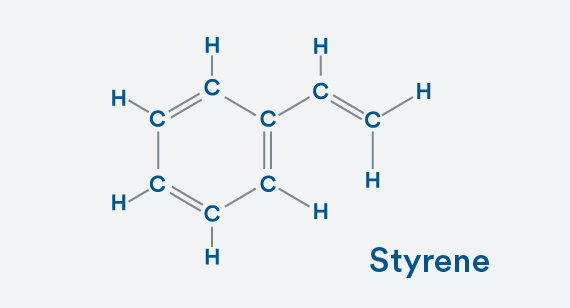
To make this styrene (a monomer), replace one of those hydrogen atoms with a C2H3 molecule. You still have a stable molecule because your carbons are happy with four bonds and each hydrogen has one bond.
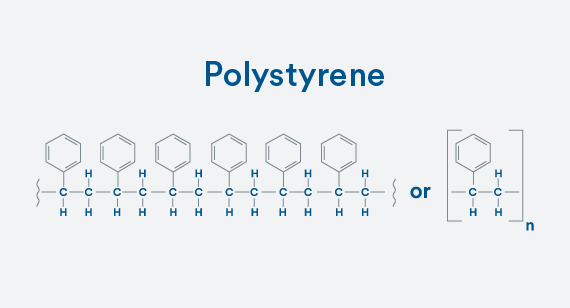
To make the homopolymer polystyrene, bond together styrene molecules. The four bonds onto carbon and one onto hydrogen still exist. It’s stable.
Making the next jump from polystyrene to ABS is a bit more complex because you need to add monomers of acrylonitrile and butadiene to the styrene. Having multiple types of monomers turns this into a copolymer. All atoms are happy with their bonds of one, four, or in the case of nitrogen, three.
Your second date is assured.
Homopolymers vs. Copolymers: Differences Between Homopolymers and Copolymers
For example, let’s compare two acetals, the homopolymer Delrin and the copolymer Celcon (aka Tecaform):
|
Characteristic |
Homopolymer |
Copolymer |
|
Crystallinity level |
Higher |
Lower |
|
Stiffness |
X |
|
|
Tensile strength |
X |
|
|
Impact resistance |
X |
|
|
Creep resistance |
Initially good |
Better in longer term |
|
Oxidation resistance |
|
X |
|
Dimensional stability |
Short term |
Long term |
|
Water resistance |
|
X |
|
Temperature resistance |
Higher heat distortion temp |
Endures exposure to long-term heat better |
It should be noted that glass-filled copolymers tend to be stronger and longer-lasting than their homopolymer relative. The added glass fibers make all the difference here to improve mechanical properties. The above differences tend to ring true for all homopolymers and copolymers within related families of monomers.
Choosing Your Polymer
In the end, what matters is that you choose the best material for your part but knowing the basic differences between homopolymers and copolymers gives you some deeper insight into which is the best to choose.

Get custom plastic parts fast. Get an online quote today.
Upload a Part